Report id’s medico-ethical issues restrict gene therapy growth
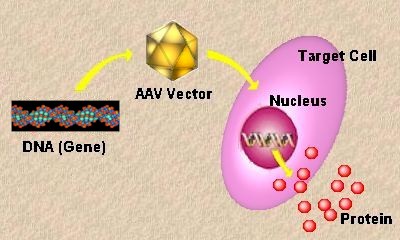 | Increasing knowledge about how to manage the risks of gene therapy is set to usher in a new era in disease therapies, with the prospect of developing treatments in treating rare, inherited, or life-threatening diseases very real. Gene therapy has gone to great pains to deal with the toxicological complications and poor therapy efficacy that have hampered the growth of this sector. |
So much so that the US gene therapies market, reveals that revenues expect to reach approximately $125 million (€106 million) in 2006 growing to approximately $6541 million in 2011.
This is not to say that gene therapy has not seen its fair share of clinical failures. Poorly regulated trials, and high levels of clinical attrition have seen the promise of gene therapy suffer from negative coverage and unsubstantiated claims.
Despite these remarkable strides, the fact remains that most biotech companies are still years away from having an approved and marketed product.
The only exception to this is the China-based company Shenzhen SiBiono GeneTech, whose product Gendicine, made news in October 2003 by becoming the first gene therapy in the world approved for marketing.
The treatment consists of an adenovirus designed to insert a gene called p53. This gene codes for a protein that triggers cell suicide when cells start to run amok, preventing them becoming cancerous.
Many tumours arise after the mutation or inactivation of p53, and in cancers of this type restoring the protein should kill the tumour cells.
The approval signals a breakthrough in the treatment of head and neck squamous cell carcinoma (HNSCC), while ongoing clinical trials expect to uncover more target cancer indications for Gendicine.
China's progress in the cell therapy market has not gone unnoticed by the west, in particular the United States. However, one could argue that US legislation and ethical wrangling concerning stem cells, a crucial factor in cell treatment, has slowed down progress, losing significant ground over rival countries.
"This is critical not only for treating the disease indication and offering hope to patients, but also to validate the whole gene therapy sector to the market and investors," said Raghunath Tantry, Frost & Sullivan's research analyst
"Companies such as Introgen Therapeutics have potentially successful late-stage pipeline products and this will add to the market momentum and increase the credibility of the technology," he added.
Introgen's INGN 241 (breast cancer) is in Phase 1-2 clinical development as is INGN 225 (lung cancer), a therapeutic vaccine, is in Phase 1-2 clinical development.
Also in Phase 1-2 clinical development are INGN 234 (a topical formulation for oral cancer prevention and treatment of oral pre-malignancies), a topical formulation for oral cancer prevention and treatment of oral pre-malignancies and INGN 401 a systemic nanoparticle tumour suppressor.
It seems that pressing concerns such as the medico-ethical issues is likely to have more of an impact on the market's fortunes than any health and safety concerns. Monitoring agencies have even questioned the ethics of testing these therapies on humans, since the science of genetics is still at a nascent stage of development.
"Successes, even minor safety and efficacy milestones, must be communicated to overcome the unwanted publicity received for toxicity and fatalities," commented Tantry.
"The market must be educated on the subtle ways to evaluate a gene therapy product for its benefits to patients that need those therapies."
By Wai Lang Chu
source: drugresearch